Electrolysis of water with 1 faraday electricity gives | CLASS 12 | ELECTROCHEMISTRY | CHEMISTRY... - YouTube
the amount of copper deposited by 1 faraday current will be maximum in an acidic solution of 1l of 1.1MCu2Cl2 2.2MCu(NO3)2 3.5M CuSO4 4.5M Cu3(PO4)3 5.10M CuF2

SOLVED:The faraday is a unit of charge frequently encountered in electrochemical applications and named for the British physicist and chemist Michael Faraday. It consists of 1 mole of elementary charges. Calculate the
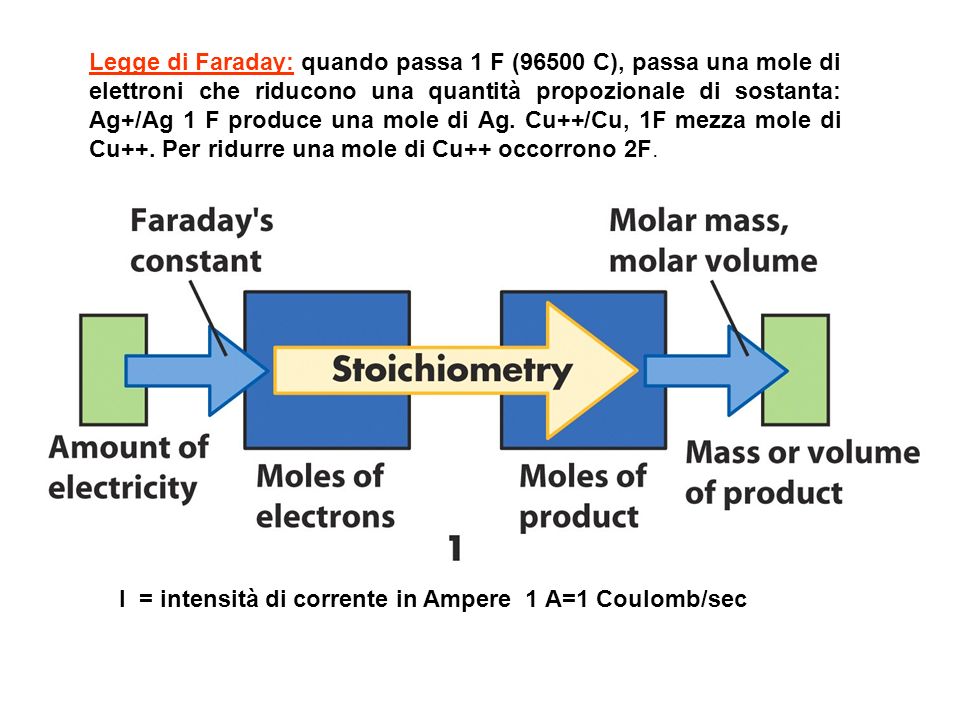
Legge di Faraday: quando passa 1 F (96500 C), passa una mole di elettroni che riducono una quantità propozionale di sostanta: Ag+/Ag 1 F produce una mole. - ppt scaricare
1.Explain and give e.g. on 'how 1 Faraday will always deposits 1 gm equivalent mass of a substance.'

The mass of the substance deposited by 1 faraday of electricity is equal to 11 grams. The value of electrochemical equivalent is: A. 11 B. 11 x 96500 - Correct Answers 11 96500 D. data insufficient

Cyber Nichel Rame 1 Faraday Tessuto EMF Schermatura 50 "x 3' Materiale di blocco del segnale - Plain Weave : Amazon.it: Casa e cucina

1 Suppose we had a 0.3A current producing 15mL of hydrogen gas in 6 min and 30 seconds. How much charge did we have per one mole of electrons? We need. - ppt download







.png)